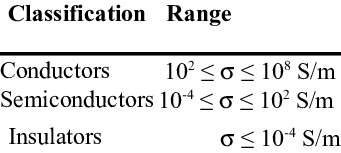
Silver has the most noteworthy electrical conductivity. Copper comes straightaway and is like silver from the perspective of nuclear construction; both having a place with a similar gathering of the intermittent table. The conductivity of copper is not exactly that of silver. Since provisions of copper are not bountiful, aluminium which is light and has a high conductivity is quickly getting more significant as a conductor material. various electrical wholesalers stockport provide all the accessories, that help people to make an easy purchase. Gold which has a conductivity higher than that of aluminium yet lower than that of silver or copper doesn’t discover use in the electrical industry since it is costly. Metals having complex designs, for example, As, Sb, Bi, Sn, Hg have lower conductivities which lie between those of ideal metal (high conductivity) and protectors (unimportant conductivities).
Variables Affecting the Resistivity of Electrical Materials
- Temperature: The electrical opposition of most metals increments with an increment of temperature while those of semiconductors and electrolytes diminishes with an increment of temperature. Numerous metals have disappearing resistivity at total zero temperature which is known as superconductivity.
- Alloying: A strong arrangement has a less normal construction than an unadulterated metal. Subsequently, the electrical conductivity of a strong arrangement amalgam drops off quickly with expanded combination content. The expansion of a limited quantity of contaminations prompts a significant expansion in resistivity.
- Cold Work: Mechanical bending of the precious stone construction decline the conductivity of metal because the confined strains meddle with electron development.
- Age Hardening: It expands the resistivity of a compound.
Movement of An Electron in An Electric Field
In a conductor, the electrons are moving about with arbitrary speed, the extent of which relies on the temperature. There are two components of the movement, as follows:
- Arbitrary movement, because of warm impacts.
- Coordinated movement, the course is dictated by the extremity of the electric field. Thus the development of electrons toward the field power prevails over that which continues the other way, the outcome being an electric current. Assessing truth that solitary quantum conditions of movement are workable for electrons, the increasing speed might be imagined as the exchange of an electron into another quantum condition of more noteworthy speed and the deceleration as the exchange of an electron into a condition of less speed. The electric flow may in this manner be treated as the prevalence of states that relate to the movement of electrons starting with one end then onto the next over the contrary states. In reality, the development of electrons among the iotas of a strong is undeniably more mind-boggling than in a vacuum and could be considered. In an entirely assembled gem, the electrons can move in almost a similar route as in a vacuum. However, gem anomaly because of pollutants and warm disturbance contorts the standard progression of charges and delivers an electric field that powers the electrons out of their underlying way. The electrons move openly just in the undistorted part of the precious stone. Here they gather energy with the speed up.